The Intersection of Genetics and Technology in Medicine
The world of medicine is undergoing a seismic shift, and at the heart of this transformation lies the dynamic interplay between genetics and technology. Imagine a future where treatments are not just one-size-fits-all but are tailored specifically to your unique genetic makeup. This isn’t science fiction; it’s the reality we are rapidly approaching. With the advent of advanced genetic sequencing technologies, we are now able to decode DNA at lightning speed and at a fraction of the cost compared to just a decade ago. This evolution is not just enhancing diagnostics but is also paving the way for personalized medicine—a healthcare revolution that promises to improve patient outcomes dramatically.
At the same time, the integration of cutting-edge technologies such as artificial intelligence (AI) is amplifying our ability to analyze genetic data. This means we can identify genetic markers that may predispose individuals to certain diseases, allowing for early interventions that could save lives. But with great power comes great responsibility. As we delve deeper into the genetic code, we must also grapple with the ethical implications of our advancements. Questions about consent, privacy, and the potential for misuse loom large as we navigate this brave new world.
Moreover, the implications of these advancements stretch beyond just individual treatment protocols. They challenge us to rethink our entire approach to healthcare. For instance, consider the way we currently treat diseases. Traditional methods often involve trial and error, leading to prolonged suffering for patients. However, with pharmacogenomics—the study of how genes affect a person’s response to drugs—we can prescribe medications that are not only more effective but also minimize adverse effects. Imagine if every time you visited the doctor, they could instantly know which medications would work best for you based on your genetic profile. This is the promise of personalized medicine, and it's not as far off as you might think.
As we stand at this crossroads, it’s essential to recognize the challenges that come with implementing these groundbreaking technologies. High costs, data privacy concerns, and the need for comprehensive education among healthcare providers are just a few hurdles we must overcome. But the potential rewards are staggering. By embracing the intersection of genetics and technology, we have the opportunity to revolutionize patient care in ways we are only beginning to understand.
- What is personalized medicine? Personalized medicine tailors healthcare based on individual genetic profiles, allowing for more effective treatments.
- How does CRISPR technology work? CRISPR allows for precise editing of DNA, enabling the correction of genetic mutations.
- What are the ethical concerns surrounding gene editing? Ethical concerns include issues of consent, potential misuse, and the long-term effects on future generations.
- What is pharmacogenomics? Pharmacogenomics studies how genes influence individual responses to drugs, enhancing treatment efficacy.
- What challenges does personalized medicine face? Challenges include high costs, data privacy issues, and the need for education among healthcare professionals.

Genetic Sequencing Technologies
In recent years, have made remarkable strides, propelling us into a new era of medicine where understanding the very blueprint of life is not only possible but also affordable. Imagine being able to read the intricate instructions written in your DNA; that’s the power these technologies bring to the table. From the early days of sequencing, which took years and cost millions, to today’s rapid and cost-effective methods, the evolution has been nothing short of astonishing. We can now sequence an entire human genome in just a few days for a fraction of the cost it used to be.
The advent of next-generation sequencing (NGS) has been a game-changer. This technology allows us to analyze multiple genes at once, providing a comprehensive view of an individual's genetic makeup. It’s akin to having a high-definition camera instead of a blurry snapshot; the detail and clarity are simply unmatched. With NGS, researchers and clinicians can identify genetic variations that may predispose individuals to certain diseases, paving the way for personalized medicine that caters specifically to the unique genetic profile of each patient.
Moreover, the implications of these technologies extend beyond just diagnostics. They play a crucial role in targeted therapies—treatments designed to target specific genetic mutations. For instance, in cancer treatment, understanding the genetic alterations in a tumor can guide oncologists in choosing the most effective therapy, much like a tailor crafting a suit that fits perfectly. This shift towards a more customized approach not only improves treatment efficacy but also minimizes the risk of adverse side effects, making the patient’s journey through treatment less daunting.
As we delve deeper into the realm of genetics, it’s essential to recognize the various types of sequencing technologies that have emerged. Here’s a brief overview:
| Sequencing Technology | Description | Key Benefits |
|---|---|---|
| Sanger Sequencing | The first generation of sequencing technology, used primarily for smaller projects. | High accuracy, ideal for validating results from other methods. |
| Next-Generation Sequencing (NGS) | Allows for massive parallel sequencing, enabling the analysis of multiple genes simultaneously. | Cost-effective, rapid processing, and comprehensive analysis. |
| Third-Generation Sequencing | Single-molecule sequencing technology that provides long reads of DNA. | Captures more complex genomic regions, useful for structural variant detection. |
As we continue to harness the power of these genetic sequencing technologies, we must also be mindful of the ethical considerations that come with them. Questions about data privacy, consent, and the potential for genetic discrimination loom large. It’s crucial that we tread carefully, ensuring that while we unlock the secrets of our DNA, we also protect the rights and privacy of individuals.
In conclusion, the evolution of genetic sequencing technologies is not just a scientific triumph; it’s a beacon of hope for the future of medicine. By decoding our genetic information, we open doors to personalized treatments that were once the stuff of science fiction. As we stand on the brink of this new frontier, the possibilities are as vast as the human genome itself.

CRISPR and Gene Editing
CRISPR technology has emerged as a groundbreaking tool in the world of gene editing, fundamentally changing our approach to genetics. Imagine having a pair of molecular scissors that can precisely cut and modify DNA at specific locations. That’s what CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) does! This revolutionary technology allows scientists to make precise alterations to the genetic code, opening up a world of possibilities in treating genetic disorders and enhancing our understanding of various diseases.
One of the most exciting applications of CRISPR is in the treatment of genetic disorders. For instance, conditions like sickle cell anemia and cystic fibrosis, which are caused by specific genetic mutations, may one day be treated by directly correcting these mutations at their source. Imagine a future where children are born free from hereditary diseases because their DNA was edited before birth. Recent case studies have shown promising results where patients have experienced significant improvements in their conditions after receiving gene therapy utilizing CRISPR technology.
The potential of CRISPR in correcting genetic mutations offers hope for patients with hereditary conditions. For example, researchers have successfully used CRISPR to edit the genes of mice with a version of muscular dystrophy, leading to improved muscle function. These breakthroughs highlight the transformative power of gene therapy. However, it's not just in animal models; human trials are underway, and the results could change the landscape of medicine as we know it.
While the promise of CRISPR is immense, it’s essential to tread carefully. The ethical implications of gene editing are vast and complex. Questions arise about consent, especially when editing embryos or germline cells that could affect future generations. Furthermore, there are concerns about the potential misuse of this technology. For instance, could it lead to a future where "designer babies" become a reality, where parents choose specific traits for their children? These questions are not just hypothetical; they are at the forefront of current discussions among scientists, ethicists, and policymakers.
As we delve deeper into the possibilities of CRISPR, we must consider the ethical landscape surrounding this technology. The ability to modify human DNA raises significant moral questions. Who gets to decide what constitutes a "desirable" trait? And what happens if gene editing leads to unintended consequences? The long-term effects of genetic modifications on future generations remain largely unknown, making it imperative to establish ethical guidelines to govern the use of CRISPR technology.
Looking ahead, the future of gene editing technologies like CRISPR promises even more exciting innovations. Researchers are continually refining these tools, making them more efficient and safer. As we enhance our understanding of the human genome, we can expect to see advancements that redefine treatment protocols, improve patient outcomes, and expand the scope of genetic interventions. The integration of CRISPR with other technologies, such as artificial intelligence, could lead to breakthroughs that we can only dream of today.
In conclusion, CRISPR and gene editing are at the forefront of a medical revolution. While the potential benefits are enormous, we must navigate the ethical complexities with care. As we stand on the brink of a new era in medicine, the decisions we make today will shape the future of healthcare for generations to come.
- What is CRISPR? CRISPR is a revolutionary gene-editing technology that allows scientists to modify DNA with precision.
- How does CRISPR work? CRISPR uses a guide RNA to locate a specific sequence of DNA, and then a protein called Cas9 cuts the DNA at that location, allowing for modifications.
- What are the ethical concerns surrounding CRISPR? Ethical concerns include issues of consent, potential misuse, and the long-term effects of genetic modifications on future generations.
- What diseases can CRISPR potentially treat? CRISPR has the potential to treat a variety of genetic disorders, including sickle cell anemia, cystic fibrosis, and muscular dystrophy.

Applications in Genetic Disorders
The advent of CRISPR technology has ushered in a new era in the treatment of genetic disorders, offering solutions that were once thought to be mere science fiction. Imagine a world where diseases caused by genetic mutations can be corrected at their source, much like fixing a typo in a manuscript. This revolutionary approach has the potential to transform the lives of millions suffering from hereditary conditions, such as cystic fibrosis, sickle cell anemia, and muscular dystrophy.
One of the most compelling aspects of CRISPR is its ability to precisely target and modify specific genes. For instance, researchers have successfully used CRISPR to edit the gene responsible for sickle cell disease, a painful and debilitating condition. In clinical trials, patients have shown remarkable improvements, with some even achieving a complete remission of symptoms. These breakthroughs highlight not just the feasibility of gene editing but also the profound impact it can have on patient outcomes.
Moreover, the potential applications of CRISPR extend beyond single-gene disorders. Researchers are exploring its use in more complex conditions, such as certain types of cancer. By modifying immune cells to better recognize and attack cancer cells, CRISPR could pave the way for innovative treatments that harness the body's natural defenses. The possibilities seem endless, and as research progresses, we may soon see a paradigm shift in how we approach disease treatment.
However, it's crucial to approach these advancements with caution. While the success stories are inspiring, the journey of gene therapy is fraught with challenges. For instance, not all genetic disorders are caused by single mutations, and the complexity of the human genome means that the solutions may not be straightforward. Additionally, ongoing research is essential to ensure that these treatments are safe and effective in the long term.
In summary, CRISPR technology holds immense promise for treating genetic disorders. With successful case studies and ongoing research, we are on the brink of a revolution in genetic medicine. Nevertheless, as we stand at this exciting crossroads, it is vital to maintain a balanced perspective, weighing the incredible potential against the ethical considerations and scientific challenges that lie ahead.
- What is CRISPR? CRISPR is a gene-editing technology that allows scientists to modify DNA with high precision, making it possible to correct genetic mutations.
- How does CRISPR work? CRISPR works by using a guide RNA to locate a specific DNA sequence, and an enzyme called Cas9 to cut the DNA at that location, allowing for modifications.
- What genetic disorders can CRISPR help treat? CRISPR has shown promise in treating disorders like sickle cell anemia, cystic fibrosis, and certain types of cancer.
- Are there risks associated with CRISPR? Yes, potential risks include unintended genetic changes, ethical concerns regarding human modifications, and long-term effects that are not yet understood.

Ethical Considerations of Gene Editing
As we stand on the brink of a new era in medicine, thanks to gene editing technologies like CRISPR, it's essential to pause and reflect on the ethical considerations that come into play. The power to modify DNA is akin to wielding a double-edged sword; while it offers the potential to eradicate genetic disorders and enhance human capabilities, it also raises significant moral questions. Who gets to decide which genes are 'desirable'? And what happens if we start editing genes for traits like intelligence or physical appearance?
One of the primary ethical concerns revolves around the concept of informed consent. In the realm of gene editing, especially when it comes to human embryos, obtaining consent is fraught with complications. How do we ensure that future generations, who cannot consent for themselves, are protected from unintended consequences? Additionally, the potential for designer babies—children engineered for specific traits—opens a Pandora's box of ethical dilemmas. Society must grapple with the implications of creating inequalities based on genetic enhancements.
Furthermore, there is the risk of misuse. The technology is powerful, but it can be misapplied. For instance, what if gene editing is used to enhance physical abilities or intelligence, leading to a new form of eugenics? The line between therapy and enhancement is increasingly blurred, and this ambiguity can lead to societal divides where only the affluent can afford genetic enhancements. As we venture further into this territory, the question of who controls this technology becomes paramount.
Another critical aspect is the long-term effects of genetic modifications. While we may see immediate benefits, the long-term repercussions could be profound and unpredictable. Changes made to the genome could have cascading effects on future generations, potentially introducing new health issues that we cannot foresee. This uncertainty necessitates a cautious approach, advocating for thorough research and the establishment of robust regulatory frameworks to oversee gene editing practices.
In light of these considerations, it's clear that while the potential benefits of gene editing are immense, we must tread carefully. Engaging in public discourse and fostering a collaborative dialogue among scientists, ethicists, policymakers, and the public is essential. Only through comprehensive discussions can we hope to navigate the complex ethical landscape of gene editing, ensuring that we harness its power responsibly and equitably.
- What is CRISPR? CRISPR is a revolutionary gene-editing technology that allows for precise modifications to DNA, offering potential treatments for genetic disorders.
- What are the ethical concerns surrounding gene editing? Key concerns include informed consent, potential misuse, the risk of designer babies, and the long-term effects on future generations.
- How can society ensure responsible use of gene editing technologies? By engaging in public discourse, establishing regulatory frameworks, and promoting transparency in research and applications.
- What is the difference between therapy and enhancement in gene editing? Therapy aims to correct genetic disorders, while enhancement involves modifying genes for desired traits, raising ethical questions about inequality.

Future of Gene Editing Technologies
The future of gene editing technologies is not just a promise; it’s a thrilling reality waiting to unfold. As we stand on the brink of a new era in medicine, the advancements in gene editing, particularly with tools like CRISPR, are set to redefine how we approach treatment protocols. Imagine a world where genetic disorders can be corrected before they manifest, where the very blueprint of life can be edited with the precision of a word processor. This is not science fiction; it’s the direction we’re heading towards.
One of the most exciting prospects is the potential for gene editing to address a myriad of conditions that have long been deemed untreatable. For instance, researchers are exploring ways to utilize CRISPR to tackle diseases such as sickle cell anemia and cystic fibrosis. By directly altering the genes responsible for these conditions, we could offer patients a chance at a healthier life. The implications are profound, not just for individuals but for society as a whole. Imagine reducing the burden of chronic diseases on healthcare systems globally!
Moreover, the integration of advanced technologies like artificial intelligence is expected to enhance the efficacy of gene editing. AI can analyze vast amounts of genetic data, identifying patterns and predicting how specific edits might affect an organism. This synergy between AI and gene editing can lead to more informed decisions, minimizing risks associated with genetic modifications. As we harness these technologies, the precision and safety of gene editing will undoubtedly improve, paving the way for more widespread clinical applications.
However, with great power comes great responsibility. As we look to the future, we must also consider the ethical implications of gene editing. Questions about consent, equity, and the long-term effects of genetic alterations on future generations loom large. It’s crucial that as we advance, we establish robust ethical guidelines to govern the use of these technologies. The dialogue surrounding gene editing must include diverse perspectives to ensure that we navigate these waters carefully.
In conclusion, the future of gene editing technologies holds immense potential to revolutionize medicine. With ongoing research and development, we can anticipate groundbreaking innovations that will not only improve patient outcomes but also expand the scope of genetic interventions. As we stand at this crossroads, let’s embrace the possibilities while remaining vigilant about the ethical considerations that accompany them.
- What is CRISPR? CRISPR is a revolutionary gene editing technology that allows scientists to modify DNA with high precision.
- How does gene editing work? Gene editing involves altering the DNA sequence of an organism to correct genetic defects or enhance certain traits.
- What are the ethical concerns surrounding gene editing? Ethical concerns include issues of consent, potential misuse, and the long-term effects on future generations.
- What diseases can gene editing potentially treat? Gene editing has the potential to treat a variety of genetic disorders, including sickle cell anemia and cystic fibrosis.
- How is AI used in gene editing? AI enhances gene editing by analyzing genetic data and predicting the effects of specific genetic modifications.

Integration of AI in Genetics
Artificial Intelligence (AI) is not just a buzzword; it’s a game-changer in the field of genetics. Imagine having a super-intelligent assistant that can sift through mountains of genetic data faster than any human could ever dream. That’s exactly what AI brings to the table! With its ability to analyze vast datasets, AI is enhancing our understanding of genetic markers and their implications for health. This integration is paving the way for breakthroughs in predicting disease susceptibility and tailoring individualized treatment plans.
One of the most exciting aspects of AI in genetics is its capability to identify patterns that might be invisible to the naked eye. For instance, algorithms can analyze genetic sequences and correlate them with clinical outcomes, helping researchers find links between specific genes and diseases. This process can lead to earlier diagnoses and more effective interventions. In fact, studies have shown that AI can improve diagnostic accuracy significantly, sometimes outperforming traditional methods.
To put it simply, the integration of AI into genetics is like having a high-powered lens that allows us to see the intricate details of our DNA. But it doesn't stop there! AI also assists in the realm of gene therapy, where it can help design more precise gene-editing strategies. By predicting how certain edits might affect cellular functions, AI can guide researchers in making informed decisions about which genetic modifications to pursue.
However, as with any technological advancement, the use of AI in genetics does come with its set of challenges. Data privacy is a significant concern; with sensitive genetic information being analyzed, ensuring that this data remains secure is paramount. Moreover, there’s the issue of bias in AI algorithms. If the data fed into these systems is not diverse, it could lead to skewed results, ultimately affecting patient care. Therefore, it’s crucial to develop AI systems that are not only powerful but also fair and transparent.
Despite these challenges, the future looks bright for the integration of AI in genetics. As technology continues to evolve, we can expect more sophisticated tools that will enhance our understanding of human genetics. Imagine a world where your healthcare provider can use AI to predict your risk of developing certain diseases based on your genetic makeup, allowing for proactive measures to be taken long before symptoms appear. The possibilities are endless!
In conclusion, the intersection of AI and genetics is transforming the landscape of personalized medicine. With ongoing research and advancements, we are on the brink of a new era where healthcare is tailored specifically to the individual, leading to better outcomes and a healthier society. So, the next time you hear about AI in genetics, remember that it’s not just a technological trend; it’s a revolution in how we understand and approach health and disease.
- What role does AI play in genetic research? AI analyzes large datasets to identify patterns and correlations that can improve understanding of genetic diseases and lead to more effective treatments.
- Are there ethical concerns regarding AI in genetics? Yes, issues like data privacy and algorithmic bias are significant concerns that need to be addressed to ensure fair and secure use of genetic data.
- Can AI predict disease susceptibility? Absolutely! AI can help predict the likelihood of developing certain diseases based on genetic information, allowing for earlier interventions.
- How does AI enhance gene therapy? AI can assist in designing precise gene-editing strategies by predicting the effects of genetic modifications on cellular functions.

Personalized Medicine
Imagine walking into a doctor's office and receiving a treatment plan that is *exclusively tailored* to your unique genetic makeup. This is the essence of , a revolutionary approach that tailors healthcare to individual characteristics, particularly one's genetic profile. The beauty of personalized medicine lies in its ability to offer *more effective treatments* while minimizing adverse effects, leading to significantly improved patient care and outcomes.
At the heart of personalized medicine is the understanding that no two individuals are the same. Just as each of us has our own fingerprint, our genetic makeup is uniquely ours. This individuality means that treatments that work wonders for one person may not yield the same results for another. Personalized medicine seeks to bridge this gap by utilizing genetic information to inform treatment decisions. For instance, when it comes to cancer treatment, oncologists can analyze the genetic mutations present in a tumor and tailor therapies that specifically target those mutations. This approach not only enhances the effectiveness of the treatment but also reduces the risk of unnecessary side effects.
One of the key components of personalized medicine is the field of pharmacogenomics, which studies how genes affect individual responses to drugs. By understanding a patient's genetic profile, healthcare providers can prescribe medications that are more effective for them, while also minimizing the likelihood of adverse reactions. For example, certain individuals metabolize drugs differently due to genetic variations, which can lead to either insufficient treatment or severe side effects. By integrating pharmacogenomics into clinical practice, doctors can avoid the trial-and-error approach that often characterizes traditional medicine.
However, the journey towards fully implementing personalized medicine is not without its challenges. High costs associated with genetic testing and the need for advanced technology can create barriers to access for many patients. Additionally, there are significant data privacy concerns surrounding the handling of genetic information. Patients must trust that their sensitive data will be protected, and healthcare professionals need to be well-versed in the ethical implications of using such information. Moreover, comprehensive education for both healthcare providers and patients is essential to ensure that everyone involved understands the benefits and limitations of personalized medicine.
Nevertheless, the potential of personalized medicine is undeniable. As research continues to advance and technology becomes increasingly accessible, we can expect to see a shift in how healthcare is delivered. Imagine a future where treatments are not only more effective but also designed with the individual in mind. This is not just a dream; it's a rapidly approaching reality that promises to transform the healthcare landscape.
- What is personalized medicine? Personalized medicine is a medical model that tailors healthcare to individual characteristics, particularly focusing on genetic makeup to improve treatment efficacy and minimize side effects.
- How does pharmacogenomics fit into personalized medicine? Pharmacogenomics studies how genes influence individual responses to drugs, allowing healthcare providers to prescribe medications that are more effective and safer based on a patient's genetic profile.
- What are the challenges of implementing personalized medicine? Challenges include high costs of genetic testing, data privacy concerns, and the need for education among healthcare professionals and patients.
- Will personalized medicine be available to everyone? As technology advances and costs decrease, personalized medicine is expected to become more accessible, but disparities in healthcare access may still pose challenges.
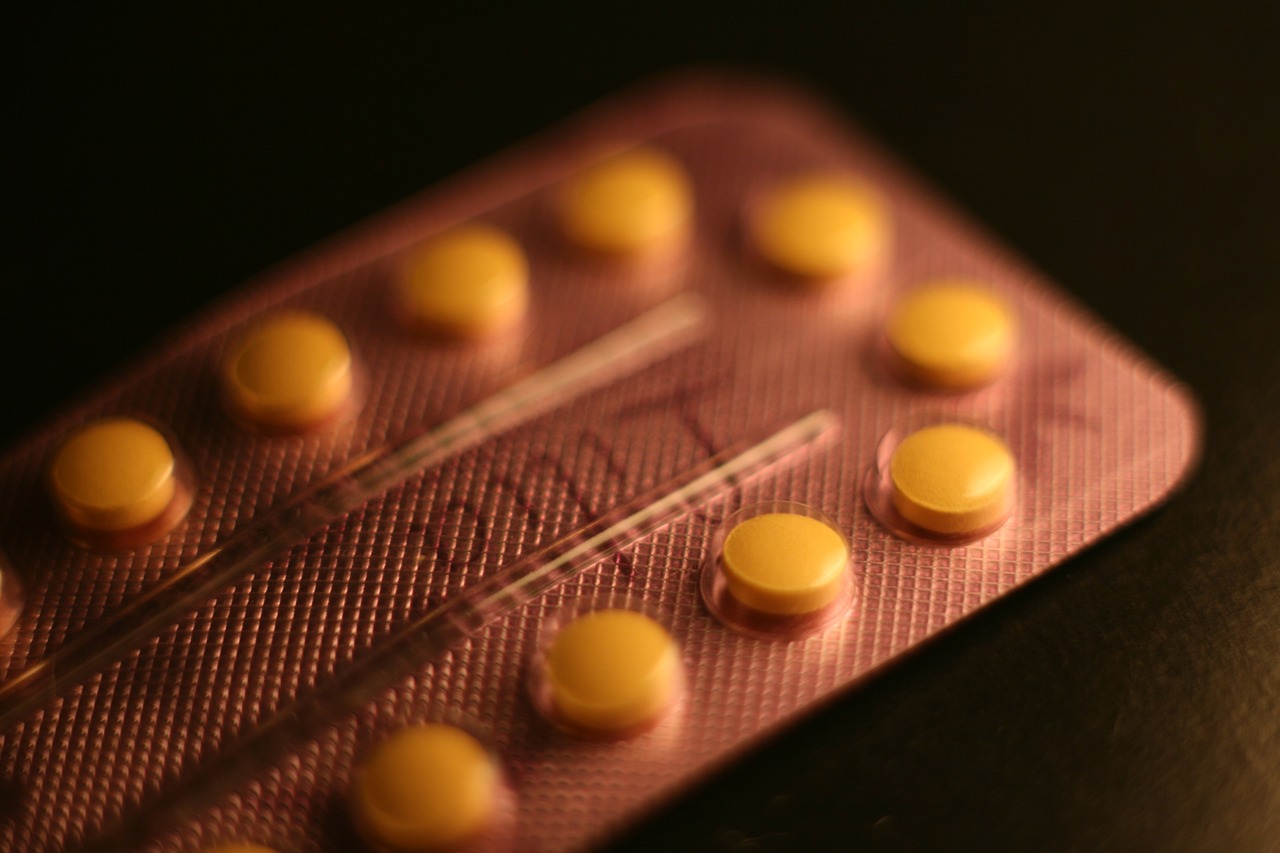
Pharmacogenomics
Pharmacogenomics is a fascinating field that dives deep into the intricate relationship between our genes and how we respond to medications. Imagine a world where your doctor knows exactly which medication will work best for you, based on your unique genetic makeup. Sounds like science fiction, right? Well, it's becoming a reality! By analyzing an individual's genetic profile, healthcare providers can tailor drug prescriptions to ensure maximum efficacy and minimal side effects. This is not just a trend; it's a **revolution** in how we approach healthcare.
At the core of pharmacogenomics lies the understanding that each person's genetic variations can significantly influence their reaction to drugs. For instance, a medication that works wonders for one person might cause adverse effects in another. This variability can be attributed to several factors, including differences in drug metabolism, efficacy, and toxicity, which are all influenced by our genetic code. By studying these variations, scientists can identify genetic markers that predict how a patient will respond to a specific drug. This is particularly crucial for medications that have narrow therapeutic windows, where the difference between an effective dose and a harmful dose is minimal.
To give you a clearer picture, let’s consider a few examples of how pharmacogenomics is making waves in the medical field:
- Warfarin: This blood thinner is used to prevent blood clots, but its dosage can vary significantly among patients. Genetic testing can help determine the right dosage based on an individual's genetic makeup, reducing the risk of complications.
- Antidepressants: Some patients may not respond to certain antidepressants due to genetic variations affecting drug metabolism. Pharmacogenomic testing can guide doctors in selecting the most suitable medication.
- Cancer Treatments: In oncology, pharmacogenomics plays a critical role in identifying targeted therapies that align with the genetic profile of a patient's tumor, leading to more effective treatment plans.
While the potential of pharmacogenomics is immense, it’s essential to recognize the hurdles we face in implementing these personalized approaches. For one, the cost of genetic testing can be a barrier for many patients. Additionally, there are concerns about data privacy and the ethical implications of genetic information. As we move forward, it’s crucial for healthcare professionals to receive comprehensive education about pharmacogenomics, ensuring they can effectively interpret genetic test results and make informed decisions about patient care.
In conclusion, pharmacogenomics is not just an emerging trend; it’s a transformative force in medicine that holds the promise of customized healthcare. As we continue to unravel the complexities of our genetic code, the future of drug therapy looks brighter than ever, paving the way for a healthcare system that’s as unique as the individuals it serves.
- What is pharmacogenomics? Pharmacogenomics is the study of how genes affect a person's response to drugs, allowing for personalized medication prescriptions.
- How does pharmacogenomics benefit patients? It helps in selecting the most effective medications with the least side effects based on an individual's genetic profile.
- Are there any risks associated with pharmacogenomic testing? While generally safe, there are concerns regarding data privacy and the potential for discrimination based on genetic information.
- Is pharmacogenomic testing expensive? The cost can vary, and while some insurance plans cover it, others may not, making it a barrier for some patients.

Challenges in Implementing Personalized Medicine
Implementing personalized medicine is like trying to solve a complex puzzle; while the pieces are becoming clearer, several challenges still hinder the complete picture. One of the most significant obstacles is the high cost associated with genetic testing and targeted therapies. Many healthcare systems struggle to provide these advanced treatments due to budget constraints, which can lead to disparities in access among different populations. Imagine having a groundbreaking treatment available, but only a few can afford it; that’s the reality for many today.
Another challenge is data privacy concerns. With the rise of genetic testing and personalized medicine comes the responsibility to safeguard sensitive information. Patients are understandably wary about how their genetic data will be used, stored, and shared. The fear of potential misuse or unauthorized access to this information can deter individuals from seeking genetic testing, which in turn limits the potential benefits of personalized medicine.
Moreover, there is a pressing need for comprehensive education among healthcare professionals and patients alike. Many healthcare providers may not be fully trained to interpret genetic tests or understand the implications of personalized treatment plans. This gap in knowledge can lead to miscommunication and ineffective treatment options. Patients, too, need to be educated about their genetic information and how it can affect their health choices. Without proper understanding, patients might feel overwhelmed or confused, which can hinder their engagement in their own healthcare journey.
Additionally, the regulatory landscape for personalized medicine is still evolving. Many countries lack clear guidelines on how to manage and implement these innovative therapies. This uncertainty can slow down the adoption of personalized medicine practices in clinical settings. For instance, healthcare providers might hesitate to recommend genetic testing if they are unsure of the legal ramifications or the reimbursement policies associated with these tests.
Lastly, the integration of technology into personalized medicine poses its own set of challenges. As artificial intelligence and machine learning become more prevalent in analyzing genetic data, there are concerns about the accuracy of these technologies. If the algorithms used to interpret genetic information are flawed, the resulting treatment recommendations could be ineffective or even harmful. It’s crucial to ensure that the technology being used is reliable and that there are checks and balances in place to verify its efficacy.
In summary, while the potential of personalized medicine is incredibly exciting, several challenges must be addressed to fully realize its benefits. From high costs and data privacy issues to the need for education and robust regulatory frameworks, overcoming these hurdles will require collaboration among healthcare providers, researchers, policymakers, and patients. Only then can we hope to unlock the full potential of personalized medicine and ensure that it is accessible to everyone.
- What is personalized medicine? Personalized medicine tailors medical treatment to the individual characteristics of each patient, particularly their genetic makeup.
- What are the benefits of personalized medicine? It allows for more effective treatments, minimizes adverse effects, and enhances patient care by considering individual genetic profiles.
- What challenges does personalized medicine face? High costs, data privacy concerns, lack of education among healthcare providers, regulatory uncertainties, and technology integration issues.
- How can patients benefit from personalized medicine? Patients can receive treatments that are specifically designed to work best for their unique genetic makeup, leading to better health outcomes.
Frequently Asked Questions
- What is genetic sequencing technology?
Genetic sequencing technology refers to methods that allow us to determine the exact sequence of nucleotides in a DNA molecule. This technology has advanced rapidly, making it faster and more affordable to decode DNA, which is crucial for personalized medicine and targeted therapies.
- How does CRISPR work in gene editing?
CRISPR is a revolutionary gene-editing technology that enables scientists to make precise changes to DNA. It uses a guide RNA to locate specific sequences in the genome and the Cas9 enzyme to cut the DNA at those locations, allowing for the addition or removal of genetic material.
- What are some applications of CRISPR in treating genetic disorders?
CRISPR has shown great promise in correcting genetic mutations responsible for hereditary conditions. Successful case studies include treatments for sickle cell disease and certain types of muscular dystrophy, showcasing its potential in gene therapy.
- What ethical concerns are associated with gene editing?
While CRISPR offers groundbreaking opportunities, it also raises ethical questions about consent, the potential for misuse, and the long-term effects of genetic modifications on future generations. These concerns highlight the need for careful consideration and regulation in the field.
- How is AI integrated into genetics?
Artificial Intelligence is increasingly being used in genetics to enhance data analysis and interpretation. AI algorithms help identify genetic markers and predict disease susceptibility, ultimately improving diagnostics and treatment strategies.
- What is personalized medicine?
Personalized medicine tailors healthcare to individual characteristics, particularly a person's genetic makeup. This approach allows for more effective treatments and minimizes adverse effects, leading to better patient outcomes.
- What is pharmacogenomics?
Pharmacogenomics is the study of how genes affect individual responses to drugs. By understanding a patient's genetic profile, healthcare providers can prescribe medications that are more effective and have fewer side effects, enhancing treatment efficacy.
- What challenges does personalized medicine face?
Despite its potential, personalized medicine encounters several challenges, including high costs, data privacy concerns, and the need for comprehensive education among healthcare professionals and patients to ensure effective implementation.



















